

Pancreatic cancer breath test trial launches nationwide, involving thousands of patients
We are proud to be investing more than £1.1M to fund the world-first breath test for pancreatic cancer, which is now to be trialled nationally on thousands of patients.
This exciting step forward follows a two-year clinical study, in which scientists at Imperial College London analysed over 700 breath samples from people with and without pancreatic cancer, as well as from those with other conditions affecting the pancreas.
Scientists are confident the “highly promising” test can detect the disease even in its earliest stages and hope that it could be used by GPs across the country within the next five years.
We are investing a further £1,141,128.35 to progress the test to a large validation trial, typically the final step before applying for regulatory approval, and then seeking adoption by the NHS.
Patients will be recruited from the NHS Urgent Suspected Cancer Pathway – under which patients should receive an appointment within two weeks of a GP referral. Around 40 trial sites are being set-up at hospitals across England, Scotland and Wales.
The breath test has the potential to revolutionise the early detection of pancreatic cancer. It is, undoubtedly, the most significant step toward a lifesaving-breakthrough in 50 years
We believe the test, a breathalyser-type device, has the potential to revolutionise the early detection of pancreatic cancer and save thousands of lives every year. The disease is the deadliest common cancer, with more than half of people dying within just three months of their diagnosis.
Vague symptoms – such as back pain and indigestion – mean that in 80% of cases the cancer goes undetected until after it has spread to other parts of the body.
No screening or early detection tests for pancreatic cancer currently exist to help doctors. Around 10,800 people are diagnosed with pancreatic cancer annually across the UK.
In future, simply breathing into a bag at a GP appointment could give many more people with the disease the chance to have surgery, currently the only potentially curative treatment.
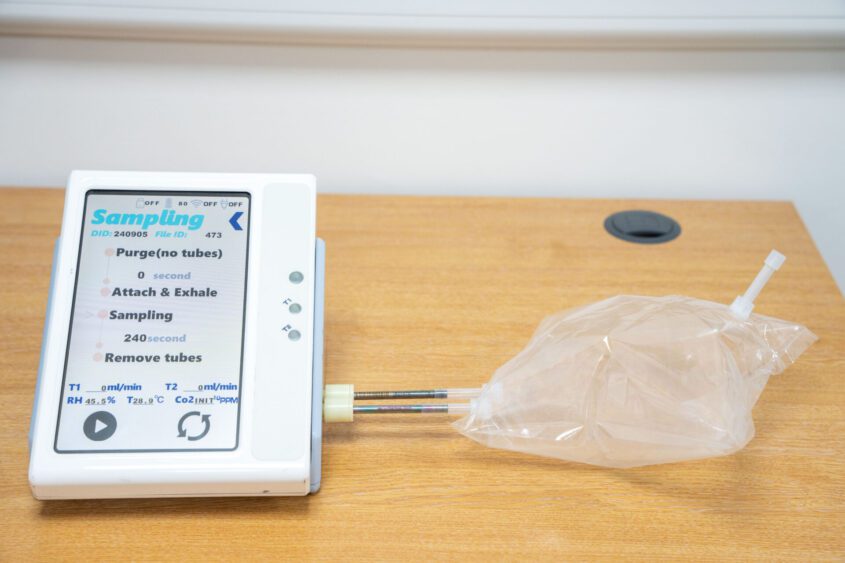
The test detects volatile organic compounds (VOCs) present in the breath. Thousands of these compounds travel around the bloodstream, are filtered out when the blood reaches the lungs, and then breathed out.
Cancer cells make different types of compounds that are detectable in the breath even at the early stages of disease. Isolating unique combinations of VOCs should enable doctors to quickly identify people likely to have pancreatic cancer and then triage them for urgent investigation.
GPs who suspect pancreatic cancer can refer patients for ultrasound, CT, or MRI scans. However, the disease’s vague symptoms are common to many much less serious conditions, meaning health professionals currently face a huge challenge in deciding who should be referred for further investigation.
The breath test, which is designed to be accurate, non-invasive, and usable in a GP surgery, could have a significant impact while remaining cost-effective for the NHS.
Breath samples can be taken in as little as 30 seconds, and scientists believe it could be possible to provide GPs with the test results within just three days. This would enable patients most at risk to be referred quickly for scans to diagnose or rule-out pancreatic cancer.
If our findings from the initial phase of the breath test study can be validated in a population of patients with an unknown diagnosis, it has huge potential to influence clinical practice and pancreatic cancer referral pathways.
The announcement of the breath test findings comes six months on from us being the official charity partner for the 2025 TCS London Marathon. Hundreds of runners took on the ultimate test to help put the first-ever into the hands of doctors. Money our amazing runners raised have helped support and progress world-class research like the breath test.
Diana Jupp, CEO of Pancreatic Cancer UK, said: “The breath test has the potential to revolutionise the early detection of pancreatic cancer. It is, undoubtedly, the most significant step toward a lifesaving-breakthrough in 50 years.
“While more years of development are still needed before we can put this exciting new technology into the hands of GPs across the country, thousands of patients with an unknown diagnosis will now help refine it in the real-world. This is the first pancreatic cancer breath test to ever reach a national clinical trial. That in itself makes this a moment of real, tangible hope.
“For decades the deadliest common cancer has been seen as too great a challenge to solve but we are determined to keep pushing the boundaries of what’s thought possible. To fully realise the potential to transform early detection, we need the government commit to increasing investment in the expected National Cancer Plan. Finding this devastating disease early gives people the very best chance of lifesaving treatment.”
Professor George Hanna’s ultimate aim is to develop a pan-cancer breath test, which could be used to detect and distinguish between a number of different gastrointestinal cancers, increasing the likelihood of its adoption by healthcare systems around the world. Early work has shown it is possible to identify unique combinations of breath VOCs for oesophageal, gastric, liver and colorectal cancer as well as pancreatic cancer.
Professor George Hanna, Head of the Department of Surgery and Cancer at Imperial College London, said: “If our findings from the initial phase of the breath test study can be validated in a population of patients with an unknown diagnosis, it has huge potential to influence clinical practice and pancreatic cancer referral pathways. The funding announced today means we can now move quickly to that patient validation study stage, which is a very exciting next step for us. We look forward to seeing how the test performs in this group of patients with suspected cancer.”



